|
Electricity is the movement
of electrons.
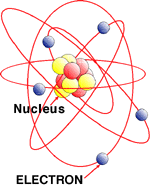 |
This is an image of an atom (the basic building
block of matter). A wire (like the kind that we use to send
electricity) is made up of lots of atoms. Electrons orbit the
nucleus (or center) of these atoms (like the moon circles the
earth), and occasionally one breaks free and flies off into
the distance. Eventually, it gets too close to an adjacent
atom, and gets sucked into an orbit around that second atom.
So an electron just got passed along the wire. We call this
movement electricity. |
If you cram electrons into one end of the wire,
and give them a way out the other end (like attaching the wire to
a lightbulb that converts electrons into light and heat), you’ve
created an electrical current. We use materials like copper for
wiring because copper atoms have ‘loose’ electrons (i.e. can easily
break free), and you can send electrons without losing too much
energy breaking them out of their orbit.
One way to think of this ‘flow of electrons’ is
to picture a person (the generator) throwing tennis balls (electrons)
over to a second person (a lightbulb).
|